Physics of metals

Table of contents
Although metals have been the basis of human civilization for centuries, it was only with the development of physics that we came to understand why they behave the way they do—both in their pure state and in the form of complex alloys. Modern materials engineering cannot exist without a deep understanding of the internal structure of metals and the mechanisms that determine their functional properties.
Metal physics is a field that studies atomic structure, the arrangement of atoms in a crystal lattice, molecular mobility, and energy transformations that occur under the influence of external factors such as temperature, mechanical stress, and magnetic fields. It is physics that explains how the arrangement of atoms translates into properties such as hardness, thermal conductivity, fracture resistance, susceptibility to deformation, and material aging processes.
Understanding the principles of metal physics enables us to consciously design and modify structural materials, endowing them with the desired properties at the microstructural level, regardless of whether the goal is to create an exceptionally light yet durable alloy for aviation or to obtain steel with increased fatigue resistance. This is why metal physics is the foundation of modern materials technology. It is not just a theory, but a practical tool that allows us to predict and control the behavior of metals in the most demanding operating conditions.

The crystal structure of metals and its significance
Metals, although they appear to be homogeneous at first glance, actually have a precisely ordered internal structure. Their atoms are arranged in regular, three-dimensional crystal lattices, which determine most of their mechanical, thermal, and electrical properties. It is this atomic order that is key to understanding how and why metals behave in a certain way.
Among the most common crystal structures in metals are the body-centered cubic (BCC) lattice, the face-centered cubic (FCC) lattice, and the hexagonal close-packed (HCP) lattice. Each of these structures is characterized by a distinct arrangement of atoms, which results in different mechanical properties. For example, metals with an FCC structure, such as copper or aluminum, are known for their high plasticity because their structure allows dislocations to move easily. In contrast, the BCC structure, characteristic of iron at room temperature, confers greater hardness but less susceptibility to plastic deformation.
The crystal structure that dominates in a given metal depends not only on its type, but also on the temperature, pressure, and history of heat and mechanical treatment. For example, iron goes through different crystal phases as the temperature changes, which directly affects its ability to harden or undergo phase transformations in steel alloys.
The crystal structure is also important in phenomena such as anisotropy, i.e., the dependence of material properties on the direction of force or conductivity. In directionally ordered metals, differences in strength along and across the crystallographic axes can be observed, which must be considered when designing structural elements.
Understanding the crystal structure is also the first step to understanding the mechanisms of lattice defects, phase transformations, and phenomena such as diffusion. It is within this ordered system that all the processes that determine the durability, functionality, and reliability of a material occur.
Atomic motion – diffusion and its consequences
Although metals are perceived as solid and rigid bodies, their atoms do not remain completely motionless. In fact, at the atomic level, matter is in constant motion – and the phenomenon that plays a key role in this is diffusion, i.e., the spontaneous movement of atoms within the crystal lattice.
Diffusion can occur both within a single type of atom and between different components of an alloy. Its intensity depends on temperature – the higher the temperature, the faster the atoms move – and on the presence of lattice defects, such as vacancies or dislocations, which facilitate the migration of molecules. For this reason, diffusion is a particularly active process during the heat treatment of materials.
One of the most significant effects of diffusion is the potential for phase transformations and homogenization of the chemical composition in metal alloys. When the material is heated, the atoms begin to move and equalize the distribution of individual elements. This enables the creation of homogeneous microstructures, which are essential for achieving the desired mechanical and technological properties.
Diffusion also plays a key role in processes such as hardening, supersaturation, aging, and carburizing, where the intended change in the composition or structure of the material occurs precisely through atomic movements. In these cases, control over the time and temperature of diffusion enables the microstructure to be shaped with high precision, which in turn translates into enhanced wear resistance, hardness, and durability of the material.
This phenomenon also has its limitations. In specific applications, such as electronic components or precision machine components, excessive diffusion can result in structural degradation, grain growth, or a loss of performance. Therefore, engineers must consciously manage this process, using it where it is desirable and limiting it where it may be harmful.
Crystal lattice defects and their technological role
Although an ideal metal crystal could be understood as an ordered lattice of atoms, real materials are never free of discontinuities. The structure of every metal contains crystal lattice defects, which, although they may seem undesirable at first glance, are of great importance for its mechanical, thermal, and technological properties.
Among the simplest but most influential defects are vacancies, i.e., empty spaces in the crystal lattice where an atom should be located. These small “holes” in the structure facilitate diffusion processes and are an indispensable element of many phase transitions. On the other hand, interstitial foreign atoms – i.e., those located in the wrong places in the lattice – can significantly affect the hardness and elasticity of the material by modifying local internal stresses.
Of particular importance are also dislocations, i.e., linear disturbances in the atomic arrangement, which play a key role in the process of plastic deformation. It is they that enable atomic layers to move relative to each other with relatively small forces, which makes metal a plastic material. The greater the number of dislocations, the easier it is for the material to deform – but at the same time, its susceptibility to strengthening through crushing increases. Skillful management of dislocations is the foundation of modern metal strengthening processes, including hardening, rolling, and precipitation hardening.
In addition to point and line defects, there are also grain boundaries, i.e., planes separating areas with different crystal lattice arrangements. Although these boundaries can be an obstacle to diffusion or current flow, they also serve as a barrier to crack propagation and dislocation movement, which makes them beneficial from a strength perspective. By controlling the grain size, for example, through heat treatment, the mechanical properties of the material can be effectively influenced.
In engineering practice, lattice defects are therefore not treated as errors, but as natural and necessary elements of the metal structure that can be used in the process of designing material properties. What was once seen as imperfections has now become a tool for precise control of microstructure.
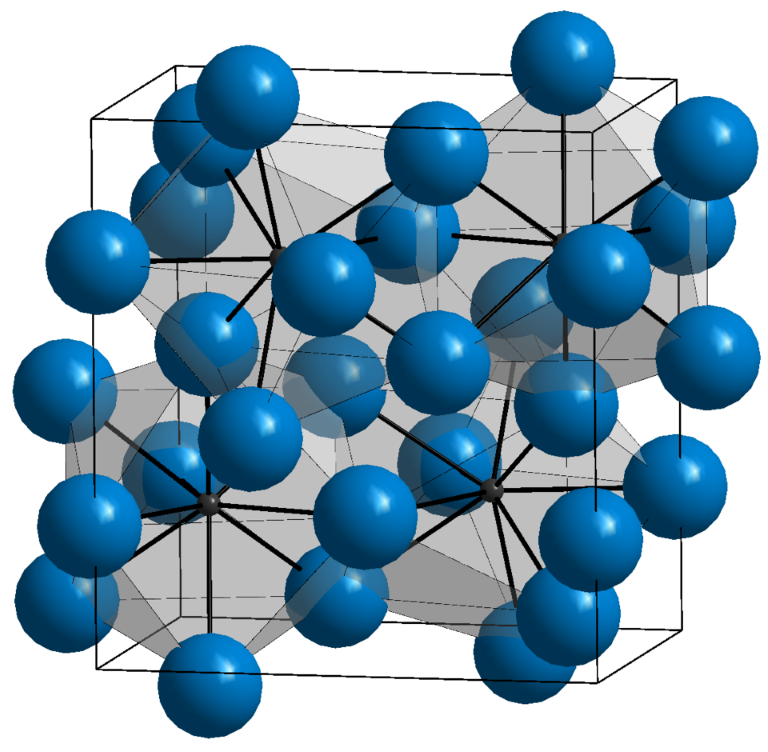
Phase transformations in metals
Metals, like many other materials, can exist in different structural states—so-called phases—which change depending on temperature, pressure, or chemical composition. These transformations, known as phase transitions, are a fundamental tool for the materials engineer, who can use them to intentionally shape the structure and properties of metals and their alloys.
A phase transition is a phenomenon in which a material changes from one ordered atomic structure to another while maintaining its overall chemical composition. A classic example of such a process is the change in the structure of iron from a spatially centered (ferrite) to a wall-centered (austenite) structure, which occurs when steel is heated. This seemingly subtle change in the arrangement of atoms leads to radical changes in mechanical properties such as hardness, ductility, and hardenability.
Phase transformations can be categorized into various types, based on their nature and mechanism. Diffusive transformations, in which atoms have time to move to new positions in the lattice, proceed relatively slowly and usually require a longer annealing time. An example of such a transformation is the formation of pearlite or bainite in steel. On the other hand, non-diffusive transformations, such as martensitic transformations, occur very quickly without atomic displacement, allowing a hard and brittle structure to be obtained in a short time. This phenomenon is at the heart of the hardening process.
In multicomponent systems, such as metal alloys, phase transformations become even more complex. Eutectoid, peritectic, and eutectic reactions occur, resulting in the formation of phase mixtures that are precisely regulated by chemical composition and thermal parameters. Understanding and controlling these reactions allows engineers to select the appropriate heat treatment conditions to obtain a microstructure with the desired properties.
It is also important to note that phase transformations are often associated with changes in volume, hardness, and thermal conductivity, which can be both beneficial and dangerous. An improperly performed transformation can lead to cracks, deformations, or residual stresses. That is why precise control of these processes, supported by a knowledge of physics and thermodynamics, is crucial in industrial practice.
Physical properties of metals in the context of their structure
The physical properties of metals, such as electrical and thermal conductivity, density, thermal expansion, and hardness, are closely related to their internal structure—both crystalline and defect-related. It is at the atomic level that it is determined how a material will behave under the influence of current, heat, or mechanical stress.
One of the most important characteristics of metals is their excellent electrical conductivity. This is due to the presence of free electrons in the crystal structure, which can move in response to an electric field. However, not all metals conduct electricity equally well – this is determined by both the type of crystal lattice and the presence of impurities and defects. For example, pure copper and silver are excellent conductors, while impurities in their structure can significantly reduce this parameter. Metal alloys, although often structurally perfect, are always poorer conductors than their pure counterparts.
The situation is similar to thermal conductivity, which is also based on the movement of electrons and lattice vibrations (phonons). Heat spreads very efficiently in metals, which is why aluminum and copper heat up so quickly and are used in heat sinks, heat exchangers, and wires. However, changes in the microstructure, such as secondary phase precipitation or grain refinement, can reduce this conductivity, limiting the rate of energy exchange within the material.
An equally important physical property of metals is thermal expansion, i.e., the ability to increase in volume as the temperature rises. This parameter is of great importance in the design of components operating in variable thermal conditions, such as pipelines, welded joints, engine parts, and aircraft components. Every material expands to a certain extent, and differences in expansion between connected parts can lead to stress and even damage.
At the intersection of physical and mechanical properties lies hardness, which is defined as resistance to permanent deformation and scratching. This property is strongly dependent on the internal structure: the presence of dislocations, grain boundaries, segregated phase particles, or internal stresses affects how easily atoms can change their position relative to each other. This is why the same steel can be soft or very hard, depending on how it has been heat-treated and the resulting microstructure.
We cannot overlook density either, which, although it may seem like a simple property, is important from the perspective of modern design. Lightweight materials such as aluminum, titanium, and magnesium alloys are gaining an advantage today wherever weight is critical—in transportation, aviation, energy, and robotics. Knowledge of density in relation to strength allows structures to be optimized in terms of weight-to-load ratio – one of the most important parameters for modern engineers.
Importantly, most of these properties are not constant – they can be modified through heat treatment, plastic working, chemical treatment, and the deliberate introduction of structural defects. This means that metals are not passive materials – they are an active medium that can be shaped not only mechanically but also physically. Knowledge of their structure allows us to control their behavior on a macro scale – and this is the essence of modern metal physics.
Physics of metals – summary
Metal physics, although often perceived as a theoretical field, is in fact the foundation of engineering practice. It is thanks to it that we understand how materials behave under the influence of temperature, stress, dynamic loads, and long-term operational processes. Without this knowledge, it would be impossible to design reliable structures, select materials with specific properties, or optimize production processes.
Knowledge of crystal structure, diffusion, lattice defects, and phase transformations allows engineers to control the properties of metals. It is physics that explains why a material can be lightweight and strong, corrosion-resistant and easy to process, as well as durable despite cyclic fatigue. In an era where materials must meet increasingly stringent requirements, both technical and environmental, metallurgy is becoming a tool of strategic importance.
Equally important is the fact that metal physics enables the creation of new materials whose properties can be programmed at the microstructural design stage. Special alloys, superalloys, functional materials, and protective coatings—all of these are created thanks to knowledge of how to manipulate structure and energy at the atomic level. In this way, metallurgical physics not only describes reality but also actively co-creates it.