Biomedical engineering in the context of materials science
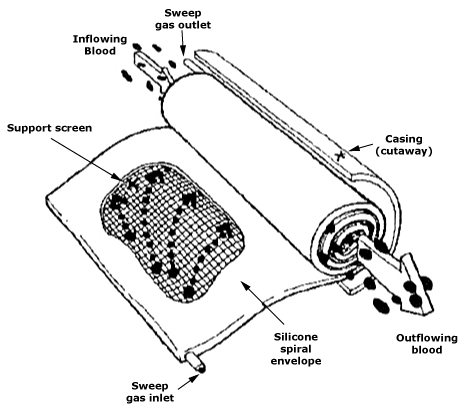
Table of contents
Biomedical engineering today is a vast ecosystem of knowledge, where materials science, fluid mechanics and transport phenomena, measurement techniques, and systems engineering intersect with physiology and clinical practice. The second edition of The Biomedical Engineering Handbook, edited by J.D. Bronzino, synthesizes these themes, demonstrating how the choice of material and its surface condition, through the design of sensors and mass flow characteristics, informs the design of artificial organs and their precision manufacturing. This article will help us see this perspective using examples from the fields of biomaterials, biomedical sensing, tissue engineering, and artificial organs, highlighting the implications for manufacturing technology and quality control.
In biomedical engineering, there are no isolated decisions — the choice of material class, surface topography and chemistry, flow and mass transfer conditions, device architecture, and manufacturing process form a systemic whole that must be designed for a specific clinical indication and biological load regime.
The place of materials science in biomedical engineering
Encyclopedically speaking, a biomaterial is a material intended for direct contact with tissue in a medical device, designed to safely and effectively replace the structure or function of the body. A broad overview of classes and design issues is provided in the section “Biomaterials,” which covers metals, ceramics, polymers, composites, biodegradable polymers, and materials of biological origin, as well as issues related to endoprosthesis maintenance and fixation. The layout of the chapters alone leads from the selection of basic classes of materials to issues of integration with hard and soft tissues, which well reflects the systemic nature of the field.
In metals (Cr–Ni–Mo steels, Co–Cr alloys, titanium alloys), corrosion and fatigue resistance, as well as the ability to precisely shape the microstructure, are key. From a practical perspective, the book emphasizes that the history of processing—smelting, plastic deformation, heat treatment, cleaning, and even final micro-machining—correlates with resistance to crevice and stress corrosion and fatigue cracking. Importantly for precision manufacturing, the chapter on metals also covers the “Manufacturing of Implants”, confirming the inseparability of material design and manufacturing technology in medicine.
Ceramics (alumina, zirconia, carbons, glass-ceramics, calcium phosphate systems) offer hardness, chemical resistance, and — in the case of bioactive glass-ceramics — the ability to form chemically mediated bonds with tissue. At the same time, the monograph reminds us of the fragility and deterioration mechanisms of ceramics, and describes bioceramic manufacturing techniques in terms of hard tissue replacement and tissue integration; it is here that the design of geometry, porosity, and surface quality meets with strict control of defects critical to fracture resistance.
Polymers are the Swiss Army knife of biomedicine, ranging from PVC and PE to PMMA and PU, and including biodegradable polyester systems, all of which possess a rich arsenal of surface modifications to enhance biocompatibility. Of particular importance is the fact that this class allows for the control of properties through molecular weight and distribution, chemical structure, and cross-linking, as well as the combination of mechanical barrier function with bioactive chemical motifs. Sterilization and its impact on properties and methods of chemogradient shaping of the protein-surface interface are also discussed, which directly translates into hemocompatibility and cell adhesion.
In composites, thanks to their structure (particles, fibers, porosity), it is possible to combine conflicting requirements: modulate anisotropy, match the modulus to bone, improve vibration damping, and achieve gradual transitions in properties. The monograph organizes these issues, discussing property boundaries, porosity, and biocompatibility. From a production perspective, controlling phase distribution and defects at the matrix-reinforcement interface becomes as important as the material itself.
The selection and qualification of a biomaterial cannot be separated from the technology used to process it and the intended method of loading. The same material with a different microstructure, topography, and surface cleanliness will exhibit a different profile of corrosion, wear, protein adsorption, and tissue integration. Therefore, in the design process, it is necessary to control the volume microstructure and the biophysical-chemical interface simultaneously.
Biomedical sensors and material-biology interfaces
Biomedical sensors serve as an interface between the biological system and the electronic system, converting geometric, mechanical, thermal, hydraulic, or chemical quantities into electrical signals. The compendium highlights a dual classification: physical sensors, which measure, among other things, muscle displacement, blood pressure, and fluid flow, and chemical sensors, which identify compounds, concentrations, and activities—from electrochemical and photometric to complex analytical systems. Optical sensors are highlighted as a highly versatile tool for both detection and transmission thanks to fiber optics.
Biopotential electrodes, which are essential in diagnostics and therapy, occupy a special place. Their operation depends not only on the electronic system and geometry, but above all on how they interact with the biological environment. Different applications—from surface Ag/AgCl to EMG needles and thin-film microelectrodes for intracardiac and central nervous system recording—require different configurations of materials, coatings, and mechanical design, as well as different qualification procedures. The problem of rejection and foreign body reactions reduces signal stability and interface durability, so it is not just a matter of electronics, but also of chemistry, surface topography, and process cleanliness.
The book also organizes the methods of applying the sensor to the patient, ranging from non-contact methods to skin contact, minimally invasive intra-body sensors, and implantable ones. Each of these methods has different requirements for biocompatibility, signal stability, and disinfection/sterilization strategies, which have direct consequences for the choice of material and manufacturing technology.
The design of a biomedical sensor is akin to designing an interface, where electrical and mechanical parameters are as critical as the choice of contact material, its coatings, surface preparation, and mounting method, which collectively determine the bioreaction and signal drift over time.
Transport phenomena and mechanical constraints
Tissue engineering and artificial organ design stem from the understanding that the transport of mass, energy, and momentum on a scale from microns to centimeters controls the function of tissues and devices. The editors of the “Tissue Engineering” section emphasize two engineering themes: the properties and development of materials on two length scales (molecular and cellular) and the analysis of rate processes. At the surface level, this involves biomolecular engineering (immobilization of adhesive ligands, control of motif density) as well as protein adsorption phenomena that shape further cellular response. At the mesoscale level, scaffolds and regeneration templates with precisely defined pore sizes, permeabilities, and degradations are key.
Furthermore, tangential stresses from flow, as well as the rates of diffusion and convection of nutrients, oxygen, and metabolites, are also involved. Chapters devoted to the influence of shear stresses on cells and the role of mass transport in tissue function show that the mechanical environment and concentration gradients translate into morphology, cytoskeletal reorganization, signal transduction, metabolite secretion, and gene regulation in endothelial cells—and thus on the development, stability, and function of neotissues. The conclusion is simple: without control of flow and mass exchange, even the best-chosen material will not perform predictably.
In tissue and artificial organ engineering, flow and diffusion parameters are just as much a design material as polymers or ceramics. Bioreactors, pore structure, and flow properties of the product must be tuned as design variables.
Tissue engineering
Tissue engineering is defined as the application of scientific principles to the design, construction, modification, growth, and maintenance of living tissues. According to Bronzino et al., for this to be possible, a cell line and cell source must be selected, a matrix-cell interface and tissue organization control must be designed, and metabolic supply must be ensured. This is where materials meet biology: immobilized adhesive ligands, protein adsorption control, and scaffold microarchitecture determine adhesion, proliferation, and differentiation.
In practice, the core consists of scaffolds with feature sizes ranging from 10 to 100 µm, which determine transport, cell colonization, and tissue growth direction. From this perspective, the class of biodegradable polymers is crucial: the chapter on biodegradable polymers describes both glycolide- and lactide-derived aliphatic polyesters and alternative families, as well as the modeling of their degradation. The author emphasizes two advantages: the disappearance of the chronic foreign body reaction as resorption progresses and the ability to serve as temporary scaffolds for tissue regeneration.
Collagen materials, as systems of tissue origin, offer a biologically familiar extracellular matrix architecture. The section on collagen describes both the chemical structure and physicochemical properties, as well as the technologies for producing membranes, porous foams, gels, and composites. Additionally, it outlines the design criteria for resorbable collagen implants, including porosity, apparent density, hydrophilicity, permeability, and in vivo stability. These parameters, which are also manufacturing process parameters, including drying rate, freeze-drying conditions, cross-linking agents, and reagent purity, directly translate into biological outcomes.
At the intersection of material and flow mechanics, devices and bioreactors emerge. Capillary fiber and microcarrier systems enable metabolism to be sustained at the cell densities required for tissue reconstruction, provided that shear stresses and concentration gradients are controlled. The same logic applies on a clinical scale when the scaffold is colonized in situ: perfusion, diffusion, and mechanical constraints of the host determine the fate of the implant.
Artificial organs and substitution medicine
The section “Prostheses and Artificial Organs” shows that organ function substitution can be bridging (e.g., extracorporeal circulation), intermittent and repeatable (hemodialysis, CAPD), or long-term with implantation. At the same time, the authors honestly point out the limitations: like any machine, an artificial organ has a limited service life due to friction, wear, and material aging in the warm, humid, and corrosive environment of the body. The balance of benefits, therefore, depends on the combination of the expected service life of the device, the method of its servicing/replacement, and the prognosis for the patient’s condition. This engineering realism coexists with a demographic fact: millions of patients are alive thanks to pacemakers, valves, dialysis, or drainage systems.
The example of an artificial kidney is particularly instructive: it is a device that embodies mass transport. In dialysis, it is the membranes, permeability coefficients, clearances, and flow conditions, as well as pharmacokinetics and the adequacy of the procedure, that determine the clinical effect. Each of these variables is related to materials science (chemistry and membrane architecture), fluid mechanics (laminar/turbulent flow, wall phenomena), and operational reliability. Hence, there has been intensive development of hemodiafiltration, surface modification, and new membrane polymers.
In the cardiovascular system, a conflict exists between the requirements of hemocompatibility and mechanical durability. Valve design involves both hemodynamic evaluation (pressure drops, energy losses, backflows, and stagnation areas) and issues of thrombus deposition and cyclic durability. In vascular grafts, thrombosis and neointimal hyperplasia are combated by introducing modifications to materials and geometry that affect shear stress distribution and flow characteristics. Any change in material or surface topography is not merely cosmetic, but rather an intervention in the biology of thrombogenesis and wound healing.
Biohybrid organs, on the other hand, are devices that incorporate living elements and combine transplant technologies with synthetic structures, promising functions closer to nature while imposing material and process requirements. This is a field in which tissue engineering and mass replacement apparatus are pulling the rope in one direction: toward increasingly functional and resilient interfaces.
Precision manufacturing and quality control
How and from what a product is made determines its final state. In metals, processing methods—such as rolling, forging, heat treatment, cutting, as well as cleaning and passivation—determine the texture, grain size, residual stresses, and surface layer composition that control fatigue, corrosion, and cell adhesion. For steel, Co–Cr and Ti alloys, the monograph discusses not only properties but also the specifics of implant manufacturing, bridging the gap between materials engineering and technology. This transition from data sheet to process sheet is absolutely critical in medicine.
In ceramics, the choice of synthesis and sintering methods, control of phase fractions and defects, and surface treatment translate into fracture resistance and bioactive capabilities. In glass-ceramic bioceramics and hydroxyapatites in particular, precise control of composition, crystallinity, and porosity is directly related to tissue integration and compressive strength. These are areas where the metrology of porosity, topography, and defects becomes part of clinical safety.
In polymers, the process determines the material’s properties, including its thermal-mechanical history, annealing environment, choice of sterilization, and stabilizing additives, which all influence degradation and aging. The monograph draws attention to two areas: surface modifications (physical and chemical) as a tool for enhancing biocompatibility and protein/cell adhesion, and the creation of chemically gradient surfaces that enable the study and manipulation of biological behavior. From a manufacturing point of view, this means that surface preparation—such as plasma, silanization, and grafted layers—should be a validated, repeatable process operation, not an art.
In tissue engineering and biohybrid organ manufacturing, technology encompasses the construction of scaffold microarchitectures, methods for sterilizing them without compromising biological function, and control of bioreactor parameters and cell colonization procedures. When designing immunoprotective capsules or open architectures, it is necessary to control diffusion, permeability, and wall mechanics simultaneously.
Material classes
Applying the above principles to clinical indications, in orthopedics, the choice of bearing pair and endoprosthesis fixation strategy involves a compromise between wear, mechanical and fatigue stability, and biological integration with the bone. This is why there has been intensive development in the literature of porous, bioactive coatings and surface modifications of ultra-high molecular weight polymers. In dentistry, a similar logic is applied to implants and restorations, where bioinert and bioactive ceramics, as well as titanium/zirconia, are combined with the need for micro- and nano-textures that promote osseointegration and the biological tightness of the mucosal transition. In the cardiovascular system, the design of valves and grafts is a direct derivative of hemodynamics and the procoagulant consequences of local geometry and roughness.
In the kidney area, membrane devices demonstrate how the theory of clearance, permeability, and total transport translates into a real-world treatment regimen, drug pharmacokinetics, and assessment of dialysis adequacy—and how slight differences in membranes, flows, and hydraulics result in clinically significant differences. Translation to the clinic requires mapping physiological requirements to material, geometric, transport, and manufacturing parameters; there is no best material outside the context of a specific application and loading.
The editors of the monograph point to the direction of evolution: devices that integrate living components with synthetic structures, such as biohybrid organs and informational prostheses, which provide the body with replacement or modulated signals to correct a medical condition. This is not only a matter of control algorithms, but also of materials and surface interfaces that must function predictably for years. The development of soft robotics devices and sensors with high biological specificity (enzyme-substrate, antigen-antibody, ligand-receptor) requires designers to master micro- and nano-manufacturing technologies, as well as the stabilization of active layers.
Biomedical engineering in the context of materials science – summary
The second edition of The Biomedical Engineering Handbook provides a comprehensive roadmap of biomedical engineering, encompassing classes of biomaterials, sensors, and measurements, as well as tissue engineering and artificial organs. Three themes recur throughout this roadmap. The first is systemicity: material, surface, transport, fluid mechanics, electronics, and manufacturing process are inseparable and together determine safety and effectiveness. The second is scalability: from molecules and adhesive ligands, through 10–100 µm architectures, to entire devices, parameters on one scale must be consistent with requirements on others. The third is manufacturability: sterilization, cleanliness, porosity and roughness control, passivation, and surface modifications are not add-ons, but elements of the design.
As a result, the biomedical designer acts as a conductor—coordinating materials, flows, signals, and processes to trigger the desired biological response and achieve operational stability collectively. This perspective, consistently developed in the monograph, remains a current foundation for practitioners in biomedicine, dentistry, and precision manufacturing.
Bibliography
Bronzino, J.D. (ed.). The Biomedical Engineering Handbook. Second Edition. CRC Press, Boca Raton, 2000.