Biomaterials in medicine and dentistry
Table of contents
Biomaterials are the backbone of modern reconstructive medicine and dentistry. Their applications range from heart valves and joint endoprostheses to intraosseous implants, sensors, electrodes, and drug delivery systems. This article aims to provide a concise but comprehensive overview of their definition, biocompatibility requirements, primary classes, mechanisms of interaction with the body, testing, and degradation principles, as well as to highlight key aspects of surface engineering and precision manufacturing that determine the clinical success of a product. The article is based on the classic monograph “Biomaterials Science: An Introduction to Materials in Medicine,” edited by B.D. Ratner et al. (Academic Press).
Biomaterials should always be analyzed in the context of their clinical application, within a specific biological environment, and after undergoing actual manufacturing and sterilization processes, as these stages define their functional properties and safety.

What is a biomaterial, and what is biocompatibility?
In the terminology adopted in the literature, a biomaterial is an inanimate material intended to interact with a biological system in a medical device. Its clinical acceptability depends on biocompatibility, i.e., the ability to elicit an appropriate, deliberately desired host response in a given application, i.e., one that enables the intended therapeutic effect and does not generate unacceptable risk. These definitions, established in the works of D.F. Williams and in the introductory chapters of monographs, among others, have become the cornerstone of the interdisciplinary development of the field.
Biocompatibility is a material–device–patient–application relationship; the same material may be biocompatible in one product and problematic in another.
Historically, biomaterials have included both metals used in prosthetics (e.g., gold in dentistry) and materials such as glass or wood in external prostheses. Today’s understanding of the discipline has developed in tandem with the crystallization of the scientific community, symposia, and the establishment of scientific societies; at the same time, the structure of professional literature and standards has taken shape.
The systematics of materials used in medicine and dentistry includes: metals, polymers, hydrogels, bioresorbable materials, ceramics and glass-ceramics, natural materials, composites, as well as thin layers, coatings and surface grafts, textiles and functional materials (actively responding to stimuli). This is summarized in Part I of the monograph, and detailed chapters discuss the characteristics, properties, and applications of individual classes.
In clinical practice, the material rarely occurs on its own but forms a medical device with a specific geometry, roughness, purity, and processing history; for example, chapters in parts II and VII describe vascular implants, electrodes, sensors, dental and orthopedic implants, and drug delivery systems.
Metals (e.g., Cr–Ni–Mo steels, Co–Cr alloys, titanium alloys) remain the basis for mechanically loaded implants (plates, screws, endoprostheses, valve components). Mechanical properties, fatigue life, and corrosion resistance are paramount, and their level strongly depends on the processing history (from smelting through plastic working to heat treatment) and microstructure.
Polymers (from thermoplastics to elastomers and cross-linked resins) provide flexibility, low density, ease of forming, and the ability to modify microstructure and function (e.g., hemocompatible coatings, conductive gels, membranes). The chapters on polymers emphasize the importance of molecular weight (Mn, Mw), polydispersity, and tacticity for mechanical and processing properties. From an operational point of view, the glass transition (Tg), crystalline melting (Tm), and rubber plateau, visible in DMA tests, are critical.
Ceramics and glass-ceramics, ranging from oxides (such as alumina and zirconia) to bioactive glasses, are crucial in applications where high hardness, chemical stability, and bioactivity are required, e.g., in restorative dentistry and bone defect repair. These materials can form a strong, chemically mediated bond with tissue (bioactivity), which is discussed in detail in the class “bioceramics“.
Natural materials (collagen, elastin, polysaccharides) are gaining importance in tissue engineering due to their similarity to the extracellular matrix and their potential to promote regeneration (e.g., skin or nerve scaffolds).
The choice of material is linked to the anatomical location and stress regime: heart valves require resistance to fatigue and blood clotting; hip endoprostheses require high strength and appropriate friction pairs; dental implants require biointegration and biological tightness of the soft tissue passage. Chapter VII provides detailed descriptions of these requirements for specific types of products.
Volume and surface properties
In biomaterials, we distinguish between volume properties (strength, modulus, impact strength, creep, fatigue, and conductivity) and surface properties (surface energy and chemistry, charge, topography, and the boundary layer after protein adsorption). The latter determines the first contact with blood and tissues — the cascade of protein adsorption, cell activation, and the coagulation cascade. The monograph includes chapters devoted to protein adsorption and the blood’s response to the material, as well as correlations between surface properties and biological response.
In polymers, the architecture of macromolecules (linear, branched, or cross-linked), crystallinity, tacticity, and molecular weight distribution determine the mechanical and thermal responses. From an engineering perspective, the parameters controlling processing (e.g., injection, extrusion, or reactive molding) and behavior during the product life cycle are critical. Mn/Mw, Tg, and Tm distributions, as well as DMA spectra, serve as the basis for designing membrane structures, coatings, and implantable elastomers.
Even subtle differences in surface properties (chemistry, nanotexture, sterilization history) can radically alter the protein adsorption profile, and subsequently affect thrombogenicity and healing, which is why managing the surface condition is as important as selecting the chemical composition of the material.
How does the body respond to biomaterial?
After implantation, a chain of host reactions is triggered, including hemostasis, acute inflammation, proliferation, and tissue remodeling in the presence of a foreign body. This results in a foreign body reaction, characterized by the chronic activation of macrophages, the formation of foreign body giant cells, and the development of a fibrous capsule. Separate, complex processes involve contact with blood (platelet activation and the coagulation cascade) and the immune system (immunology and the complement system). The compendium of chapters 4.1–4.7 provides a conceptual framework and methods for evaluating this response.
The response pattern depends on the implantation site, size, and roughness, as well as material composition and surface condition; even the same material can induce different macrophage phenotypes in soft tissue and periosteal tissue. Therefore, the assessment of biocompatibility must be application-specific (in vitro + in vivo).
Biomaterial testing encompasses in vitro studies (cells, proteins, and hemocompatibility), in vivo studies (animal models and implant sites), blood–material tests, and the design of animal models tailored to the clinical indication. The guidelines compiled in sections 5.1–5.5 establish a common language for academic and industrial laboratories, as well as for regulatory assessment. Results from one level (e.g., in vitro) cannot be directly extrapolated to clinical results; triangulation of data (in vitro–in vivo–explant analysis) is the gold standard in product development and oversight.
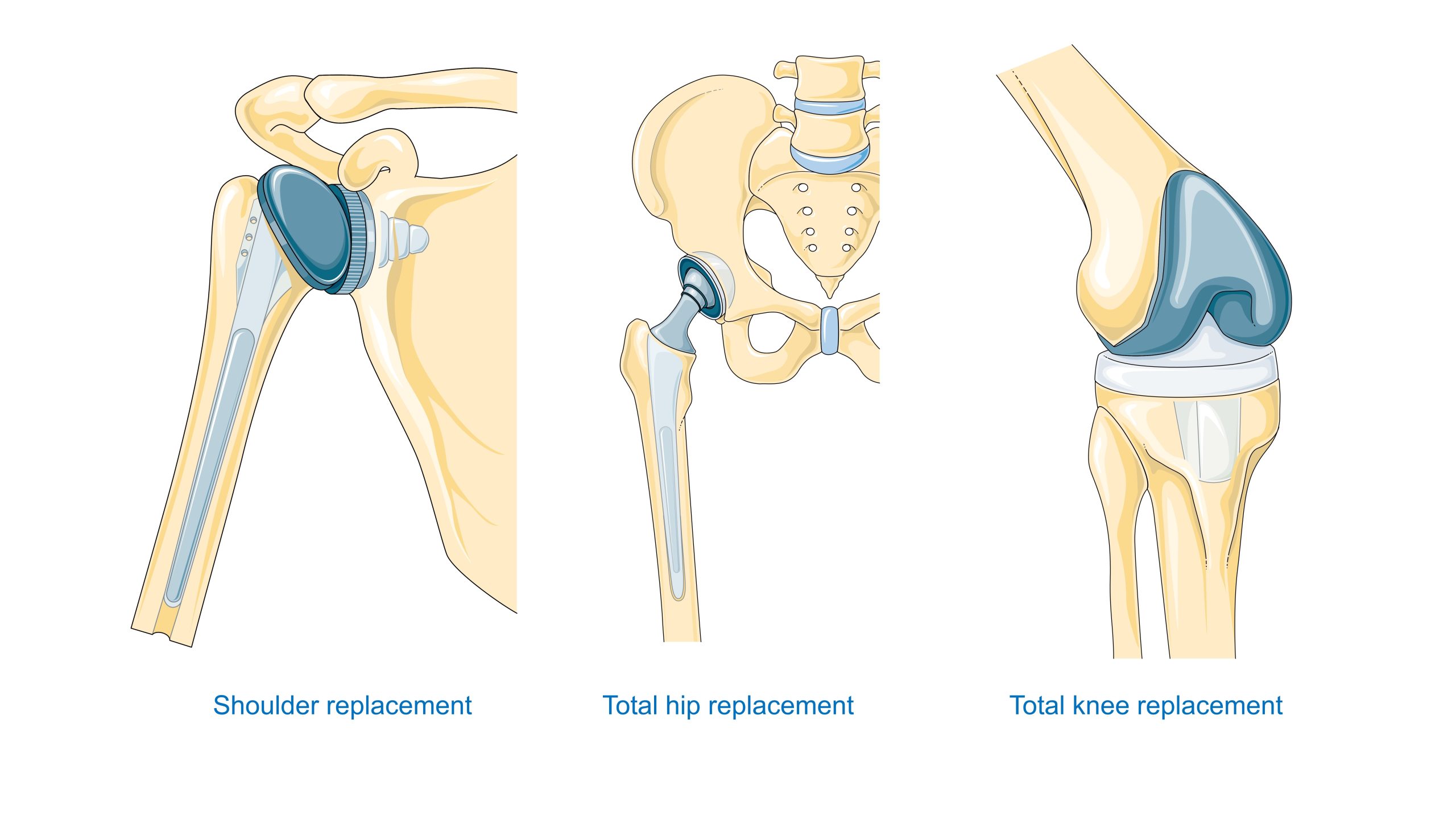
Degradation and aging in a biological environment
The body is not a simple environment for material design. It is rich in proteins, enzymes, and cells capable of generating reactive oxygen and chlorine species. In this environment, materials are subject to hydrolysis, oxidation, corrosion, fatigue, stress cracking, and even pathological mineralization. Sections 6.1–6.5 provide a cross-sectional overview of the degradation mechanisms of polymers, metals, and ceramics.
Particularly instructive is the mechanism by which the host polymer oxidizes. In acute inflammation, neutrophils produce superoxide anion, hydrogen peroxide, and (with the participation of myeloperoxidase) hypochlorous acid (HOCl); macrophages, which dominate chronically (along with giant cells), sustain the emission of free radicals, and MPO adhesion to the foreign body surface may provide a catalyst at the implantation site. This environment promotes the oxidation of ether and urethane bonds, as well as the initiation of cracks and stress cracking in urethane elastomers, phenomena observed, among others, in polyurethane components of electrodes and pacemaker leads.
Degradation is synergistic: alternating loads produce microcracks and a fresh, reactive surface; water absorption alters the local pH and facilitates the diffusion of reagents; hydrolysis products enhance hydrophilicity and the penetration of degradation agents. Controlling composition, antioxidant stabilization, and residual stress conditions is critical for long-term safety.
In corrosive metals, degradation includes pitting and crevice corrosion, stress corrosion, and tribological wear in friction pairs, which can lead to loose particles and tissue reactions. Ceramics, although chemically more stable, are brittle and sensitive to crack-initiating defects; therefore, their design requires rigorous control of defects.
Examples of clinical applications
Cardiovascular system. Materials for valves, grafts, stents, extracorporeal systems, and artificial organs must strike a balance between hemocompatibility and mechanical durability, as well as resistance to protein/cell deposition. Non-thrombogenic strategies include surface modifications (such as heparinization and hydrogels) and control of surface energy.
Dentistry – Titanium and zirconium implants have revolutionized prosthetic rehabilitation. Success depends on biointegration with bone (more precisely, close adhesion and mechanical retention) and the tightness of the passage through the mucous membrane. Materials for restorations (precious ceramics, glass-ceramics) benefit from advances in bioceramics and precision machining.
Orthopedics – Hip and knee endoprostheses require a compromise between static and fatigue strength, wear resistance, and the tribological properties of the friction pair (metal–UHMWPE polyethylene, ceramic–ceramic, metal–metal). Additionally, the connection with the bone is crucial: comparing acrylic cement to porous coatings for osseointegration.
Precision manufacturing, microstructure, and surface engineering
In the case of metal implants, the properties result from the entire processing chain, from smelting and refining through plastic deformation and heat treatment to machining, welding, coating, and sterilization. Microstructure (grain size, phases, texture) and process-induced defects influence fatigue strength and corrosion resistance. The introduction of porous coatings and structures (e.g., sintered meshes, plasma sprays) is an example of an interdisciplinary task: the adhesion, stiffness, topography, and fatigue integrity of the entire component must be balanced.
In polymers, processing parameters (temperature, time, and residual stresses) are equally important, as are the selection of antioxidant stabilizers and purity control, as these factors determine the subsequent biostability. In practice, there have been cases of stress cracking in polyurethanes used in pacemaker leads, specifically at the interface between residual stresses, the biological environment, and oxidation by the host.
Surface engineering is a set of tools for modifying properties, ranging from physicochemical coatings to the application of thin films and grafted polymer layers, as well as micro/nano topography. Correlations between surface parameters and biological response are the subject of dedicated practical chapters.
For long-term implanted devices, small process decisions (e.g., type of sterilization, storage aging conditions) can become major causes of clinical differences years later; documenting processing history and quality control are integral parts of biomaterial design.
Ethics, regulations, and standards
Products are brought to market under rigorous evaluation systems, including the FDA and ISO. The cost of demonstrating safety and efficacy is significant, but it protects patients and shapes quality policy. At the same time, ethical questions arise: how to balance patient interests and economic pressures, how to design studies with minimal risk, when and how to withdraw obsolete solutions. These issues, along with the role of consensus standards and technological development, are discussed in the section on new products and standards. Clinical progress requires simultaneous progress in assessment methodology, standards, and ethics; otherwise, innovation may be illusory or risky.
Prospects
On the horizon are biologically functional (stimulus-responsive) materials and advanced biosensor and artificial organ systems (implantable and extracorporeal) that make the bio-material interface the center of physiological informatics. Their success will depend on subtle control of surface interactions, long-term stability, and protection against degradation. The section of the monograph devoted to artificial organs and biosensors shows how closely materials, electronics, and biology must work together. The future of biomaterials lies in hybrid systems, which will require even more meticulous control of microstructure and surface.
Biomaterials in medicine and dentistry – summary
Biomaterials are active components of therapy that co-create the biological microenvironment. Success is determined equally by the choice of material class, surface engineering, microstructure, and processing history, as well as an understanding of host response and degradation mechanisms over time. Metals, polymers, and ceramics bring different sets of advantages and risks; natural polymers and bioactive systems expand the palette with regenerative functions. From a clinical perspective, each project presents a multi-objective optimization problem for a specific indication and patient population, which the monograph systematizes, progressing from definitions and properties, through contact biology and testing, to the practical aspects of implantation and explant analysis. Finally, precision manufacturing and quality control are not the last stage, but an integral part of biomaterials science, as they dictate the long-term behavior of the material in the body.
The overarching competence in biomaterial design is the ability to combine materials science with biology and product engineering—from chemistry and microstructure, through surface, to manufacturing and sterilization. This results in a single, well-documented clinical solution, verified in the in vitro–in vivo–clinical pathway.
Bibliography
Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E. (eds.). Biomaterials Science: An Introduction to Materials in Medicine. Academic Press, San Diego–London–Boston–New York–Sydney–Tokyo–Toronto, 1996.